|
What
is a Photon: Particle or Wave?
D. M. Marett (2010)
Part
1
The
question I try to answer herein is, how long is a photon? What I have
uncovered is that there is a general time t
that appears to be characteristic for the emission of a photon (or the
absorption of a photon), from the beginning of the process until the
end, and in that time, the energy (hf) of one photon is emitted. If a
photon was a point particle, the emission time should be no longer than
its physical length. So how long is it? This depends on its frequency -
an RF photon can be many kilometres in length. The characteristic
length of a visible light photon is on the order of cm's to meters.
This should pose a serious problem to anyone considering a photon a
"point particle", or a particle of any kind for that
matter.
Evidence:
1)
How a photon is created or absorbed.
Henderson et.al. (1)
describes how during a transition from the 2S to the 1S orbital, the
charge density oscillates back and forth between the two orbitals at
the Bohr frequency, which is:
Fig.1:
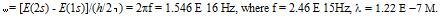
This periodic fluctuation is called
“transient nutation”. The number of cycles from start to finish is
between 106 and
107. The oscillations begin small,
are strongest in the middle, and then die off, as shown below:
Fig.2:
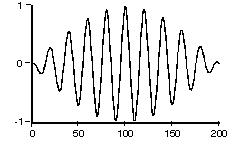
This should effectively be the form of a
photon in space. The effective length for a S2 to
S1 transition photon should be approximately: 12.2 cm to 122 cm
(average = 61cm) based on their estimates.
The above authors (1) also discuss how physics students are
taught that the transition is effectively instantaneous - quote:
"
The state of affairs has been greatly influenced by over 40 years of
popular belief that since a bound system exhibits only certain discrete
energies and a transition from one to another cannot proceed through
any observable intermediate levels, then the corresponding wave
function must also evolve in a similar discontinuous manner. This interpretation has been shown to be incorrect.
"
They go on to say:
At
this point, the natural questions of the student are, "How is a photon
created or absorbed? What is the mechanism of this process and how long
does it take?" The usual instructor response may be that a transition
involves a quantum jump, which is an instantaneous process and the
Uncertainty Principle prohibits us from observing or describing in
classical terms the details of the transition, or he/she may evade the
question by claiming the concepts are beyond the scope of an
introductory course and will be developed later in quantum physics or
physical chemistry. After completing a bachelor's
degree, our
student has been exposed to a lot of the prescriptive formalism of
quantum mechanics with heavy emphasis on finding eigenvalues and
solutions to the time-independent Schrödinger equation and possibly
modest exposure to the time-dependent equation and perturbation theory
for the purpose of developing transition probabilities. However, to
her/his great disappointment, freshman questions probably still remain
unanswered."
They then explain how the actual
transition has been observed in detail:
The
first experimental measurements of bulk samples undergoing
spectroscopic transitions were obtained from nuclear magnetic resonance
observations of the transient nutation effect (5) and spin echoes (6, 7)
using coherent radiation produced by a single radio frequency
oscillator. More recently, the analogous transient nutation effect (8, 9) and so called "photon echoes" (10-12)
have been observed in molecular spectra using pulsed coherent laser
radiation. These experiments confirm that there are no "quantum jumps"
in the non-stationary state; rather there are smooth, continuous
periodic changes in the magnetic and electric properties of a system
undergoing a transition.
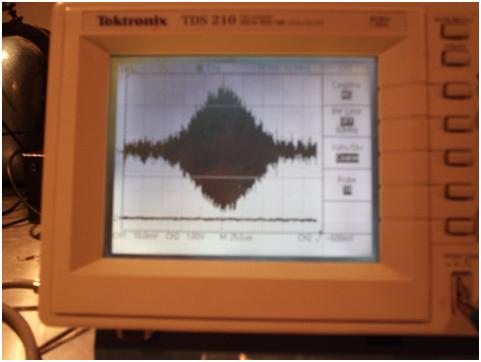
Fig.
3 is a spin echo of a glycerol H1 sample ( 10mV resolution, 25uS per
division) , from the home-built NMR (18 MHz) described on this website (2). Notice
that the transient nutation (spin echo) is around 125 us in duration.
This puts the length of the EM emission from the relaxing ensemble of
protons in the glycerol sample at greater than 37,500 meters, since 125
us x the speed of light = 37.5 km travelled from the start to the
finish of the transition. Another
paper (3) discusses the length of a stimulated
nutation echo from a microwave transition electron spin echo at 5.95
GHz. This is found to be around
40 us long (or around 12 km from start to finish).
Fig.3:
Spin Echo from a glycerol H1 sample at 18 MHz.
2) Radiative Lifetime:
The
radiative lifetime of an excited electronic state e.g. in a laser gain
medium is the lifetime which would be obtained if radiative decay via
the unavoidable spontaneous emission were the only mechanism for
depopulating this state. It is given by the equation.
Fig. 4:
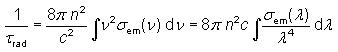
which
shows that high emission cross sections and a large emission bandwidth
inevitably lead to a low radiative lifetime. This is because the cross
sections describe not only the strength of stimulated emission but also
that of spontaneous emission.
Another important aspect is that a
shorter mean wavelength of the emission implies a shorter radiative
lifetime. This results from the
increased mode density of the radiation field. A
consequence is that ultraviolet lasers tend to have a higher threshold
pump power than infrared lasers.
The
radiative decay rate of a classical
electron oscillator is given by :
Fig.5:
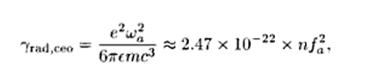
where
n is the refractive index of the medium in which the oscillator is
embedded, and the oscillation frequency is measured in Hz. A useful
rule of thumb is that the purely radiative lifetime of a classical
oscillator is approximately given by:
Fig. 6:
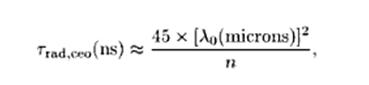
where
n is again the refractive index of the medium, and lo is the free
space wavelength in um. By
way of example, if at lo
= 500 nm (visible region) , the classical oscillator radiative lifetime
is about 11 ns (about 3.3 meters from start to finish). .
The Fig. 6 equation is used to calculate
the photon length at different classical oscillator emission wavelengths
as shown in the table below:
|
Wavelength
(m)
|
radiative
lifetime(s)
|
Photon
Length (m)
|
Photon
Energy
|
spectrum
|
|
9.93609E-07
|
4.44267E-08
|
1.33E+01
|
0.001
|
KeV
|
IR
|
|
4.96805E-07
|
1.11067E-08
|
3.33E+00
|
0.002
|
KeV
|
VIS
|
|
2.48402E-07
|
2.77667E-09
|
8.33E-01
|
0.005
|
KeV
|
UV
|
|
1.24201E-07
|
6.94167E-10
|
2.08E-01
|
0.01
|
KeV
|
UV
|
|
6.21006E-08
|
1.73542E-10
|
5.21E-02
|
0.02
|
KeV
|
UV
|
|
3.10503E-08
|
4.33854E-11
|
1.30E-02
|
0.04
|
KeV
|
UV
|
|
1.55251E-08
|
1.08464E-11
|
3.25E-03
|
0.08
|
KeV
|
UV
|
|
7.76257E-09
|
2.71159E-12
|
8.13E-04
|
0.2
|
KeV
|
UV
|
|
3.88129E-09
|
6.77897E-13
|
2.03E-04
|
0.3
|
KeV
|
UV
|
|
1.94064E-09
|
1.69474E-13
|
5.08E-05
|
0.6
|
KeV
|
x-ray
soft
|
|
9.70322E-10
|
4.23686E-14
|
1.27E-05
|
1.3
|
KeV
|
x-ray
soft
|
|
4.85161E-10
|
1.05921E-14
|
3.18E-06
|
2.6
|
KeV
|
x-ray
soft
|
|
2.4258E-10
|
2.64804E-15
|
7.94E-07
|
5.1
|
KeV
|
x-ray
soft
|
|
1.2129E-10
|
6.62009E-16
|
1.99E-07
|
10.2
|
KeV
|
x-ray
hard
|
|
6.06451E-11
|
1.65502E-16
|
4.97E-08
|
20.5
|
KeV
|
x-ray
hard
|
|
3.03226E-11
|
4.13756E-17
|
1.24E-08
|
40.9
|
KeV
|
x-ray
hard
|
|
1.51613E-11
|
1.03439E-17
|
3.10E-09
|
81.9
|
KeV
|
x-ray
hard
|
|
7.58064E-12
|
2.58597E-18
|
7.76E-10
|
163.8
|
KeV
|
x-ray
hard
|
|
3.79032E-12
|
6.46493E-19
|
1.94E-10
|
327.6
|
KeV
|
x-ray
hard
|
|
1.89516E-12
|
1.61623E-19
|
4.85E-11
|
655.1
|
KeV
|
x-ray
hard
|
For
the values shown, the length of a photon from the start of the emission
to the finish varies from around 13 meters long in the IR region, to
around the size of an electron orbital in the hard x-ray region. The
higher one goes in x-ray frequency, the more compact and discrete the
EM emission, and the closer it will fit into the space occupied by the
electron orbital.
The Length of a Photon considered
in relation to the laser emitting it:
Now
consider a photon being emitted by a HeNe laser at 632.8 nm
wavelength. If we assume a distance between the laser mirrors
of
say 20 cm and that the atom emitting the photon is near the back
mirror, we would be forced to conclude that the photon, being 5.4
meters long by the time the atoms has completed its emission, would
have changed direction by reflection off the laser mirrors about 27
times! How can we consider a photon in this laser to be a particle when
it is travelling simultaneously along opposite directions in 27
different paths?
Conclusions on the Length of a Photon
The
above discussion suggests that because of the finite time required for
the emission of an EM wave of energy E = hf from an oscillating source
that the length of this putative photon must be so long as to make it
ludicrous to consider it a point particle. In the RF region, a photon
would be many kilometres in length. In the IR region, a photon would be
several meters in length, and in the visible region it would be many cm
to meters in length. It is only in the hard x-ray region that the
"photon" would approach the dimensions of an orbital, but would still
be about 1000x longer than the "point electron" that it interacts with.
This evidence calls into question the assertions in the literature that
a "photon" should be considered a particle in any real sense.
Wave-particle duality is a common feature of modern physics - even
though these two concepts are mutually exclusive. A wave is a form of
motion, a particle is an object. You can have a wave in a population of
particles, but a single particle can't be a wave in and of itself. As
has been said elsewhere on this website, one needs to take a stand -
either light is a wave and space is a medium, or light is a particle
and space is empty. Feynman at least took a stand when he said that
light is a particle-
"I
want to emphasize that light comes in this form - particles. It is very
important to know that light behaves as particles, especially for those
of you who have gone to school, where you were probably told something
about light behaving as waves. I am telling you the way it does behave,
like particles."(4) This must be the wrong stand, since the
shear
length of the photon should rule out a
particulate nature. If
there is a contradiction, it is symptom of the inadequacy of the
theories that have been offered to us.
Footnotes:
1) Henderson, Giles,et.al., "How a Photon is
Created or Absorbed" http://jchemed.chem.wisc.edu/JCEWWW/Articles/
2) Home-built 18 MHz Nuclear Magnetic
Resonance(NMR)Spectrometer (2008)
3) http://arxiv.org/ftp/physics/papers/0203/0203039.pdf
4) Feynman , QED, P. 15.
|